
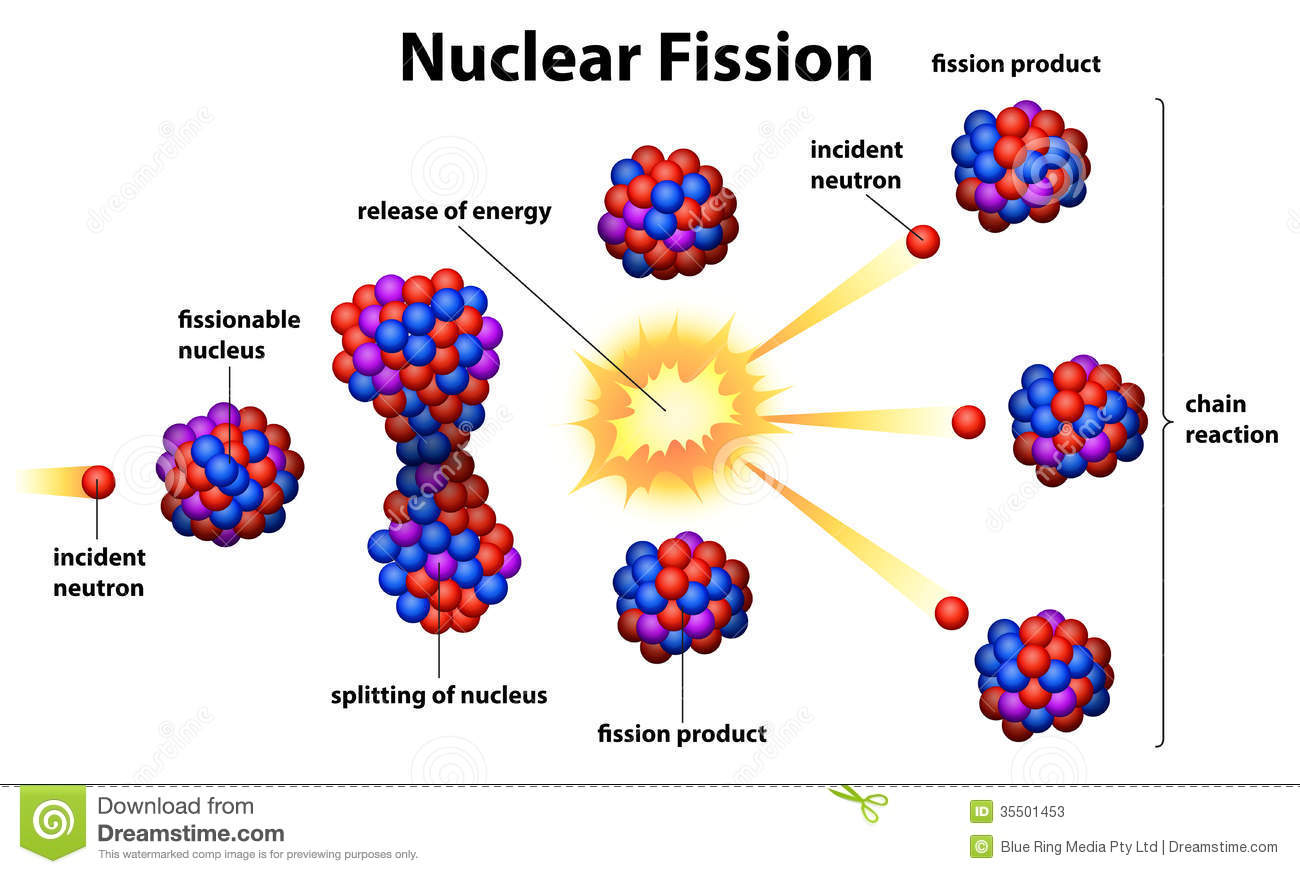
It is simply called "hex" in the industry.Įnriched uranium is uranium in which the 235U isotope concentration has been increased to greater than the 0.711 percent 235U (by weight) currently present in natural uranium.ĭepleted uranium or "DU" is uranium in which the 235U isotope concentration has been decreased to less than 0.711 percent. UF 6 is the compound of uranium used for the two most common enrichment processes, gaseous diffusion enrichment and centrifuge enrichment. Uranium hexafluoride (UF 6) is a white solid which forms a vapor at temperatures above 56 degrees Centigrade. Uranium tetrafluoride (UF 4) is known as "green salt" and is an intermediate product in the production of uranium hexaflouride. Uranium ore is rock containing uranium mineralization in concentrations that can be mined economically, typically 1 to 4 pounds of uranium oxide per ton or 0.05 to 0.20 percent uranium oxide. It is sometimes confusingly called "yellowcake" but this is not a standard name. Yellowcake typically contains 70 to 90 percent uranium oxide (U 3O 8) by weight.Īmmonium diuranate is an intermediate product in the production of yellowcake, and is bright yellow in colour. It takes its name from the color and texture of the concentrates produced by early mining operations, despite the fact that modern mills using higher calcining temperatures produce "yellowcake" that is dull green to almost black. Uraninite is the most common ore of uranium. Some Important Materials Containing Uranium Weapon", because only the nuclei participate. Name for both this and the hydrogen bomb ( nuclear fusion) would be "nuclear The first atomic bomb worked with by this principle ( nuclear fission). Nuclei, a nuclear chain reaction occurs, and if there isn't anything toĪbsorb some neutrons and slow the reaction, it is explosive. If these neutron are absorbed by other 235U upon bombardment with slow neutrons, its 235U isotope becomes the very short lived 236U, that immediately divides into two smaller nuclei, liberating energyĪnd more neutrons. Uranium was the first element that was found to be fissile, i.e. The artificial 233U isotope is also fissile and is made from 232 thorium by neutron bombardment. The isotope 238U is also important because it absorbs neutrons to produce a radioactive isotope that subsequently decays to the isotope 239Pu ( plutonium), which also is fissile. The isotope 235U is indispensable for both nuclear reactors and nuclear weapons because it is the only isotope existing in nature to any appreciable extent that is fissile, that is, fissionable by thermal neutrons. Its two principally occurring isotopes are 235U and 238U. gamma (body-centered cubic) from 774.8☌ to melting point - this is the most malleable and ductile state.beta (tetragonal) stable from 667.7 C to 774.8☌.alpha (orthorhombic) stable up to 667.7☌.Uranium metal has three allotropic forms: Its melting point is 1132☌, its density 19050 kg/m 3. Uranium is a heavy, naturally radioactive (it doesn't have stable isotopes), metallic element, in the periodic table uranium has the symbol U and atomic number 92.


 0 kommentar(er)
0 kommentar(er)
